Imagine, after years of research you and your team discover an unfortunate mutation in the mouse model you have been using. The work you have dedicated yourself to for so long must be discarded and you need to start over. This is the harsh reality for anyone breeding laboratory mice – whether you’re in academia, the pharmaceutical space or working with a mouse vendor – one day you wake up and “surprise” spontaneous mutations have occurred producing phenotypes that can be mistakenly attributed to the intended experimental mutations. Perhaps the most painful consequences of not using a genetically stable mouse line is that years down the road you may have to reinterpret results from earlier, seemingly sound experimental designs when these spontaneous mutations are finally discovered.
This is exactly what happened to Harvard University’s Dr. Shiv Pillai and his research team. As described in their recent paper, “Striking Immune Phenotypes in Gene-Targeted Mice Are Driven by a Copy-Number Variant Originating from a Commercially Available C57BL/6 Strain”, just published in Cell Reports online on May 19, 2016. Years’ worth of the team’s data studying the effects of two genes on hematopoiesis had to be re-evaluated when they discovered a spontaneous mutation in the inbred strain that they had used for the initial backcrossing.
Experimental Reproducibility- An Inevitable Roadblock
Their odyssey started approximately ten years ago when Dr. Pillai’s group began studying the effects of sialic acid acetylesterase (Siae) and cytidine monophospho-N-acetylneuraminic acid hydroxylase (Cmah) genes, that seemed to influence B cell signaling and immune tolerance. Their conclusions were based on experiments using Siae and Cmah knockout (KO) mice that had been backcrossed to the inbred C57BL/6 substrain, C57BL/6NHsd between 2005 and 2008. They hit a major roadblock, however, when years later they further backcrossed their Siae KO model to JAX’s C57B6L/J genetic background. Suddenly the immune phenotype previously attributed to Siae, disappeared. According to personal communications with Dr. Pillai, these unexpected results started what turned out to be an over 48 month-long quest to figure out when, how, and why their experiments went wrong.
Failure to replicate previous work is, understandably, both a puzzling and daunting experience in the face of the ever-present pressures to publish, secure grant funding, and propel research programs forward. Every biomedical researcher can relate to Dr. Pillai’s frustration at spending both precious grant money and their researchers’ time to solve unexpected problems, instead of advancing their research.
Uncovering the Root of the Problem
It took incredible tenacity and skills, including genetic crosses, SNP arrays, and whole-genome sequencing of C57BL/6 strains from multiple vendors for the primary author Dr. Mahajan, Dr. Pillai, and their collaborators to find the cause underlying the irreproducibility of their previous experiments and to get their research back on track. As it turned out, the team found that the aberrant immune phenotype that had previously been attributed to the absence of Siae protein was, in fact, the result of a spontaneous mutation that only occurred in the C57B6N/6Hsd substrain: a homozygous copy-number variant that disrupts the function of the Dock2 gene. This mutation was not found in B6 and B6N substrains from three other commercial providers.
The Mahajan et al. 2016 paper poignantly highlights the discouraging reality that in some cases, published conclusions may need to be revisited. What happened to these researchers, and potentially to others using C57BL/6Hsd for immunology research, is unfortunate. But animals bred with any mouse breeder are vulnerable to the side effects of genetic drift. Such mutations have been reported in substrains of all kinds and from all vendors.
Mitigating Genetic Drift- JAX Genetic Stability Program (GSP)
The sobering fact is that over time, sudden, random, and cumulative genetic drift eventually changes the phenotype of all inbred mouse strains. The impact of this drift depends on the nature of the new mutations and the research in which the strains are being used. In the end, the effect is like walking through an ever-expanding minefield: it is just a matter of time before a spontaneous mutation affects an experiment in an unexpected way (Stevens et al., 2007). Inattention to genetic drift and failing to account for it can confound research conclusions, making them inaccurate, misleading, and perhaps even unusable.
The Jackson Laboratory (JAX) is the only mouse supplier with a colony management program supported by a Genetic Stability Program (GSP) that was designed to limit cumulative genetic drift, including copy number variations, in most commonly used research strains. For more than 10 years, JAX has regularly rebuilt inbred strain foundation stocks every few generations from cryopreserved, pedigreed embryos (Taft et al., 2006).
Scientists using mice managed under the JAX GSP, including C57BL/6J, C57BL/6NJ, BALB/cByJ, NOD/ShiLtJ, and others, can be confident that the mice that they receive 25 years from now will be as genetically comparable as possible to the mice that you are working with today. This Genetic Stability Program is the industry’s gold standard to generate lab mice that remain as genetically stable as possible.
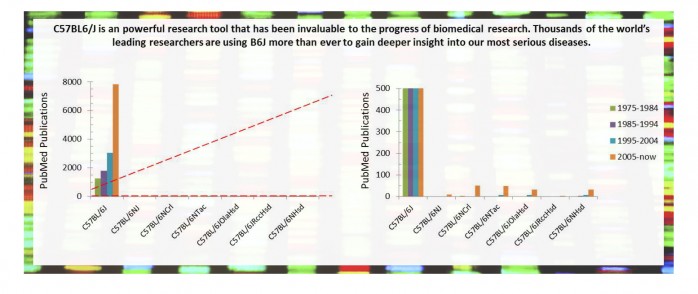
JAX strives to provide the highest quality mouse models and a large variety of GSP inbred strains to ensure the research you perform today is reproducible tomorrow. More researchers trust and utilize GSP protected B6J and B6NJ mice than ever before.
To learn more about genetic drift and the steps you can take to mitigate it attend our webinar “Genetic Drift: What it is and How to Minimize Its Impact on Your Research.” JAX Technical Information Scientists will be available live and online both during the presentation and afterward to answer any additional questions that come to mind.
Final Thoughts:
After reading the Mahajan et al. 2016 article, a friend of mine put the Harvard team’s experience in very simple terms: “Breed them, breed them, breed them, and the strain’s DNA will turn into something else.” Can you afford such a detour in your research career? A little dose of prevention now may save you from a big genetic headache several years down the road.