CRS and Bispecific Antibody Development

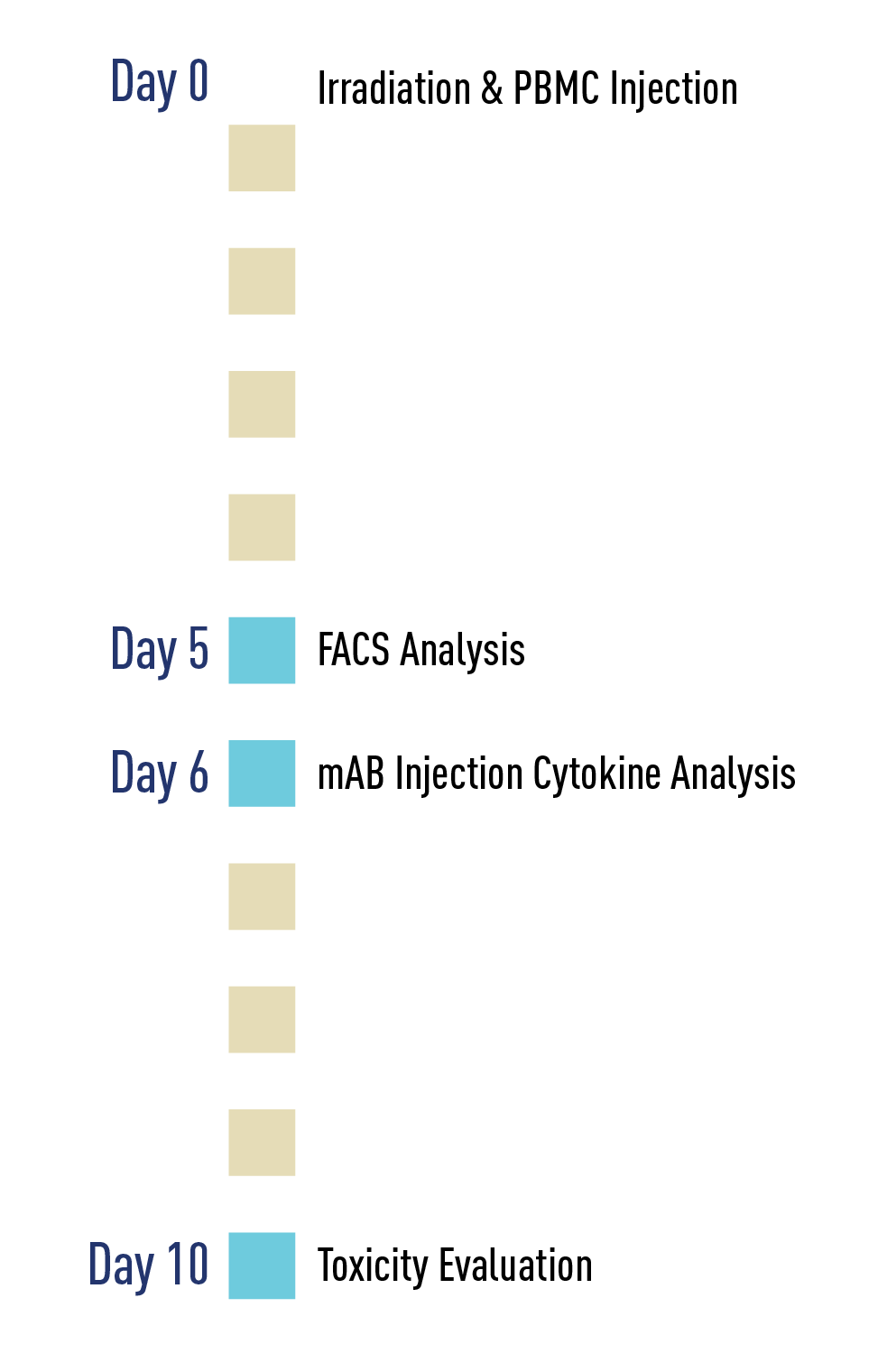
Bispecific antibodies are shown to be a promising approach to cancer immunotherapy, but CRS is a known issue when developing these therapeutics. The CRS Evaluation Studies can evaluate Bispecific T-cell Engagers (BiTEs) alone, or in combination with other therapeutics.
When evaluating bispecific drugs, it is important for a tumor antigen to be present in the model. The bispecific reaching to the tumor antigen is what triggers the cytokine release response. JAX has access to a large library of PDX Tumors that can be engrafted into the NSG models utilized in the CRS Evaluation Studies, giving researchers access to optimal tumor models and optimal translationally relevant environments.

Bispecific antibodies are shown to be a promising approach to cancer immunotherapy, but CRS is a known issue when developing these therapeutics. The CRS Evaluation Studies can evaluate Bispecific T-cell Engagers (BiTEs) alone, or in combination with other therapeutics.
When evaluating bispecific drugs, it is important for a tumor antigen to be present in the model. The bispecific reaching to the tumor antigen is what triggers the cytokine release response. JAX has access to a large library of PDX Tumors that can be engrafted into the NSG models utilized in the CRS Evaluation Studies, giving researchers access to optimal tumor models and optimal translationally relevant environments.

Bispecific antibodies are shown to be a promising approach to cancer immunotherapy, but CRS is a known issue when developing these therapeutics. The CRS Evaluation Studies can evaluate Bispecific T-cell Engagers (BiTEs) alone, or in combination with other therapeutics.
When evaluating bispecific drugs, it is important for a tumor antigen to be present in the model. The bispecific reaching to the tumor antigen is what triggers the cytokine release response. JAX has access to a large library of PDX Tumors that can be engrafted into the NSG models utilized in the CRS Evaluation Studies, giving researchers access to optimal tumor models and optimal translationally relevant environments.