
Lupus is a chronic, incurable, autoimmune disease that primarily afflicts young and middle-aged women. Worldwide, approximately 5 million women have been diagnosed with the disease, and women of African, Asian, Hispanic and Polynesian descent are particularly susceptible to it.
Lupus also is a disease that has touched the Technical Information Services group at JAX directly: in 2006, our colleague, Dr. Monica Roberts, succumbed to the disease just 4 months after her diagnosis. Monica was only 30.
35 different genes influence lupus susceptibility
Studies in both mice and human have implicated as many as 35 different genes that influence lupus susceptibility. In addition, environmental factors such as infection or exposure to sunlight are thought to trigger in some cases the abnormal immune responses that lead to disease.
Systemic lupus erythematosus
Lupus is a cagey disease in that there are several varieties and not all afflicted patients develop the same clinical manifestations. Patients with the most common type, systemic lupus erythematosus, or SLE, typically show anti-nuclear auto-antibodies in their blood that likely contribute to variable combinations of severe muscle and joint pain, rash, photosensitivity, inflammation of the tissues lining the heart and lungs, and glomerular nephritis.
Mouse models of lupus
JAX distributes three main mouse models that are making significant contributions to both furthering our understanding of the genetics that underlie lupus and identifying targets for potential therapeutics: MRL/MpJ-Faslpr/J (000485), NZBWF1/J (100008), and BXSB/MpJ (000740). The lupus-related characteristics of these models are summarized in Table 1, and also have been reviewed, briefly, in a previous blog post. In this post, I’d like to focus more on the BXSB/MpJ model.
Table 1. Phenotype comparison of JAX’s most popular lupus models
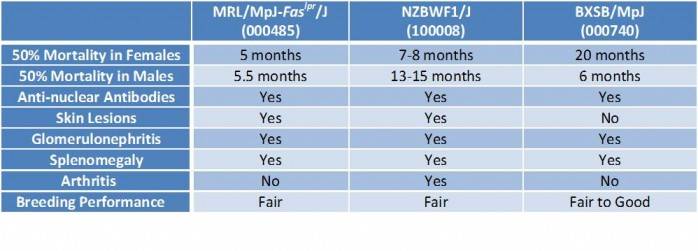
BXSB/MpJ males have a severe lupus phenotype
As in humans, lupus in NZBWF1 mice primarily affects females. In MRL-lpr mice, both females and males develop disease.
In BXSB/MpJ mice, however, males manifest a significantly more severe lupus phenotype, owing to the duplication and translocation of a 17 gene-containing region from the X chromosome onto the Y chromosome – the Yaa mutation. The increased signaling activity of Tlr7, one of the genes duplicated in the Yaa mutation, has been identified as the primary driver of lupus in this model (Subramanian S et al. 2006).
Suite of BXSB congenic mice
Because female BXSB/MpJ mice develop only relatively mild lupus-related phenotypes, BXSB/MpJ, probably, are the least popular of the three models. The model, however, should not be given short shrift just because females lack a severe phenotype.
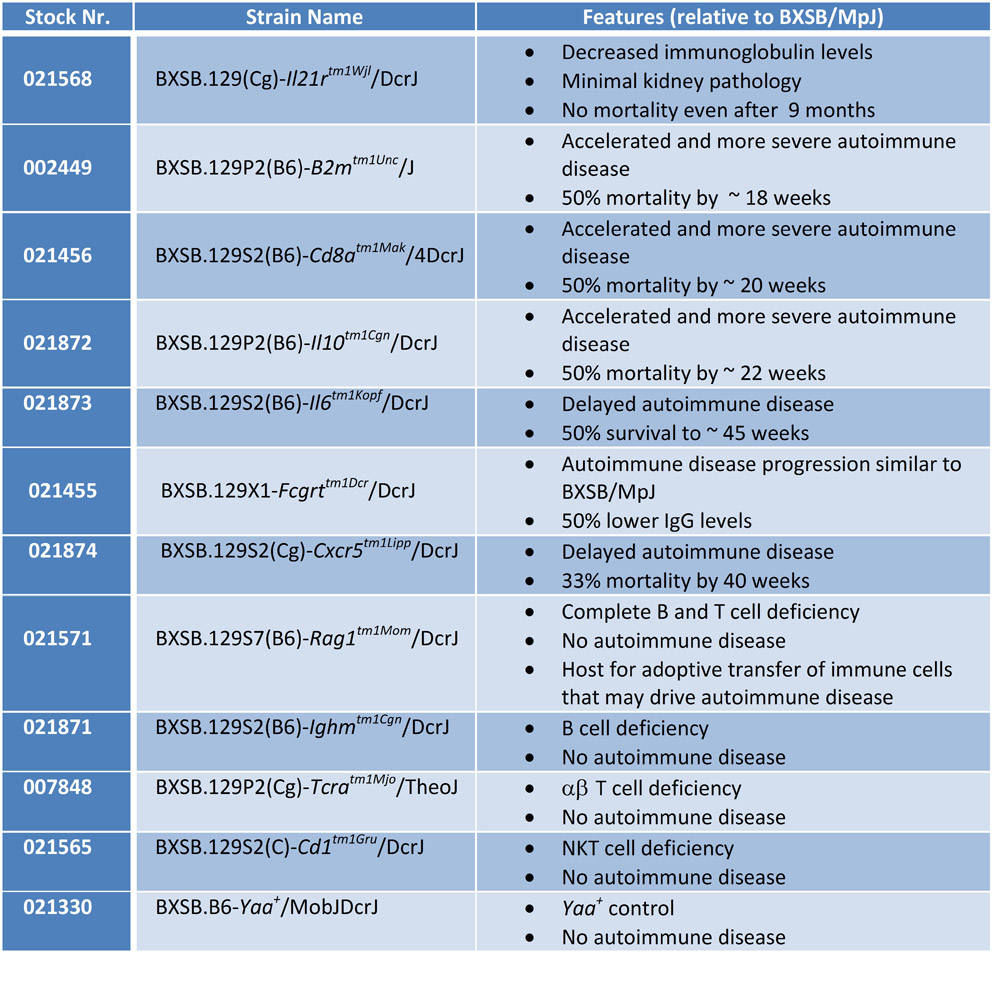
Indeed, JAX investigator Dr. Derry Roopenian and his colleagues have created a suite of BXSB congenic mice that are proving to be powerful tools in revealing the roles of specific cytokines and immune cell populations in the development of lupus in this model (see below).
For example, using BXSB congenic interleukin 21 receptor-deficient strain (BXSB.129(Cg)-Il21rtm1Wjl/DcrJ – Stock # 021568) Dr. Roopenian’s group has demonstrated that interleukin 21 (IL-21) signaling in B cells is critical for the development of lupus-like kidney disease in BXSB mice: BXSB-Il21a-/- mice and irradiated BXSB reconstituted with Il21-/- B cells develop only minimal lupus-related kidney pathology and typically survive more than 275 days (Bubier JA et al. 2009; McPhee CG et al. 2013).
In contrast BXSB MHC class I-deficient mice (BXSB.129P2(B6)-B2mtm1Unc/J – Stock # 002449) and mice deficient in other genes that affect class I-dependent pathways (e.g. BXSB.129S2(B6)-Cd8atm1Mak/4DcrJ – Stock # 021456) have revealed the importance of intact MHC class I responses in protecting BXSB males from developing even more aggressive kidney disease. Dr. Roopenian’s BXSB recently developed congenic strains, and their lupus phenotypes are summarized in Table 2.
Table 2. Phenotype of BXSB congenic mice available from JAX
These mice, and others like them, could be keys to identifying important targets for the development of new therapeutics with which to treat lupus. We can only hope that such therapies ultimately may ease the pain of those women suffering from lupus, and minimize the impact of the disease on their everyday lives.