
(L to R) JAX Professors Carol Bult and Jeff Chuang
NCI Pediatric PIVOT program aims to expedite approval of treatments for childhood cancers
Childhood cancer. As devastating as this diagnosis is for families, it may be followed by more bad news: Most of the targeted treatments available for adults with cancer are not FDA-approved for pediatric oncology patients. To address the need for increased preclinical testing and approval of pediatric cancer treatments, the National Cancer Institute (NCI), part of the National Institutes of Health (NIH), is funding the Pediatric Preclinical In Vivo Testing Program (PIVOT), a new initiative which will be coordinated by The Jackson Laboratory (JAX).
The urgency of childhood cancer drugs
“My daughter Kelsey died in my arms. Treatment options were limited and experimental trials were few and hard to come by. We missed one by a single day. I futilely think back to that trial and what may have been,” said Sean Gallagher, whose daughter, Kelsey, died in 2005, just a month shy of her 18th birthday. “The JAX PIVOT program has an ambitious and laudable goal – to accelerate the testing and approval of childhood cancer drugs. To a child, to a family, to a community, these are dire situations where time is the enemy. We can’t kill time, but with better approaches, like the PIVOT program, I have no doubt we can kill more cancer.”
Although there are approximately 200 FDA-approved agents for the treatment of cancer, fewer than 40 of those are approved for children. In addition, there are few targeted therapies available for pediatric cancers, and pediatric oncology treatments can take a long time to reach patients: The median drug approval period for these agents is six years.
Expediting pediatric cancer research
The NCI Pediatric PIVOT coordinating center, which will be run by JAX and partnering organization Seven Bridges Genomics – and funded by the NCI – serves to improve and expedite this process. The PIVOT coordinating center will connect pharmaceutical companies that are developing oncology therapies with seven testing centers around the world that have the capacity to test them in specialized cancer models derived from pediatric cancers. While PIVOT is a new program, the testing centers in the consortium have more than seventeen years previous experience working together on preclinical testing of novel pediatric cancer treatments
“This program has the potential to make new treatments more available for pediatric oncology, which has not seen advancements at the same rate as adult cancers,” said JAX Professor and Knowlton Family Chair Carol Bult, Ph.D.Bridges the digital biology divide, by integrating computation and informatics with biomedical research.Carol Bult , who coordinates the PIVOT initiative at JAX with Professor Jeffrey Chuang, Ph.D.Computational studies of cancer image and sequence data to improve treatment outcomesJeff Chuang . “By making crucial connections between pharmaceuticals and testing centers, we are improving a process that could yield lifechanging results and provide hope to the many families who experience pediatric cancers.”
PIVOT helps to address key challenges in implementation of the Research to Accelerate Cures and Equity for Children Act (RACE), which went into effect in August 2020. The legislation tackles gaps in pediatric cancer treatment development by mandating that new, targeted therapies for adult cancers also be tested in a pediatric setting when the molecular targets or mechanisms of the treatment agent are relevant to childhood cancer.
“Given the large number of agents under development for adults with cancer and the corresponding relatively small numbers of children with specific cancer types, a robust process for prioritizing at the preclinical level is an essential component for successful pediatric cancer drug development,” said Dr. Malcolm Smith, associate branch chief for Clinical Investigations Branch, Cancer Therapy Evaluation Program, NCI. “To address this need, we’re excited to have JAX playing a leadership role in our NCI-supported pediatric preclinical testing program and to have seven exceptional research teams taking the lead for testing for the childhood cancers in their areas of expertise.”
Leveraging cutting edge technology in the fight against cancer
JAX and Seven Bridges will be coordinating the work of the testing centers, which will be primarily using patient-derived xenografts (PDX) to test novel agents from pharmaceutical companies for their applicability in solid tumors and blood cancers in children.
JAX has played a major role in developing PDXs, a system in which human tumors are implanted into mice for preclinical drug testing. PDXs are one of the most realistic experimental systems for assessing the efficacy of anti-cancer agents in human tumors, and PDX testing has been a key step in clinical approval of many cancer therapeutics.
The Pediatric PIVOT coordinating center will organize, manage, and help analyze the treatment data to maximize their value for individual treatment studies and for further research. This work will be in conjunction with the NCI funded testing centers in the consortium:
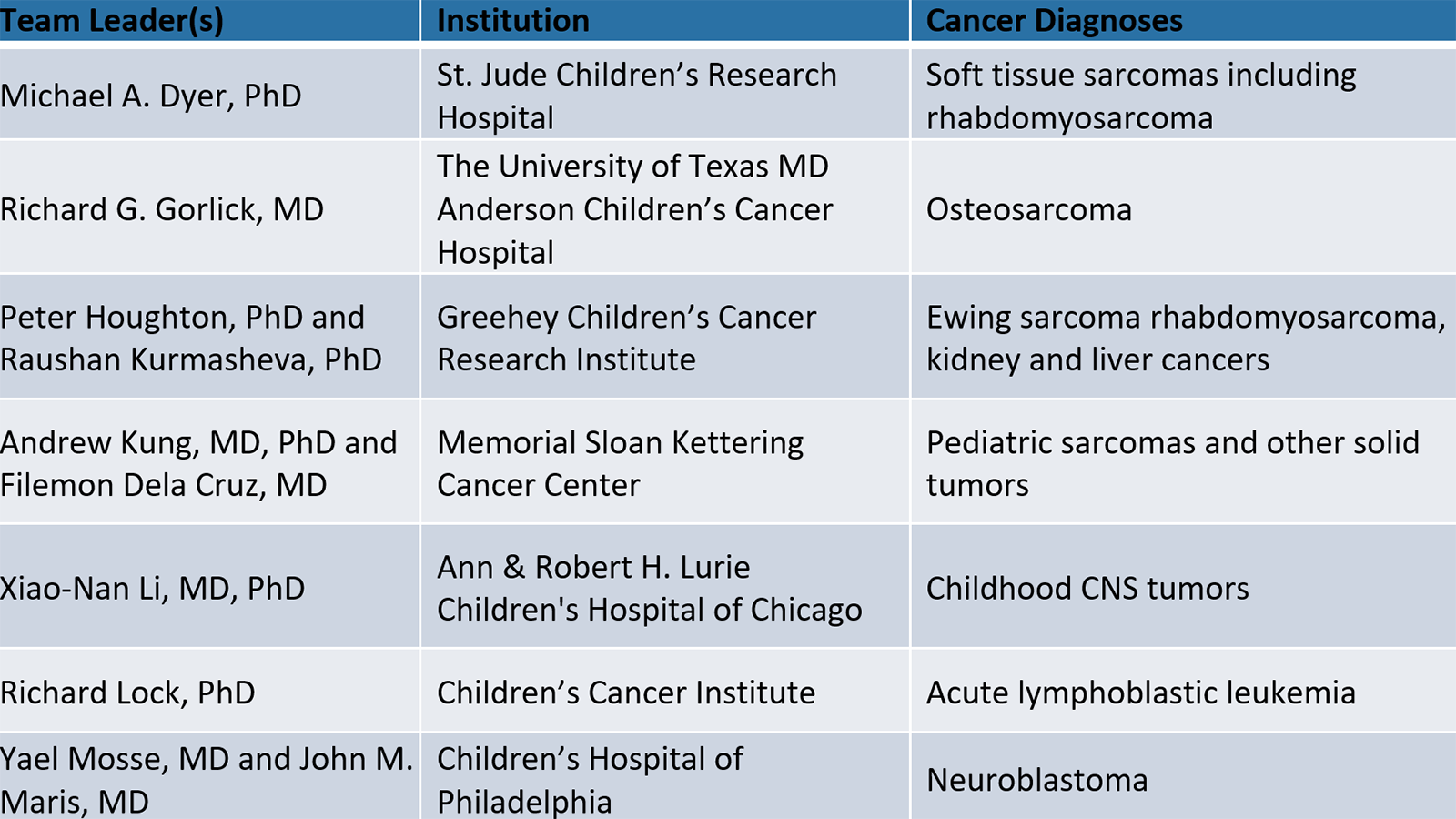
In addition to Bult and Chuang, several JAX representatives will work as consultants on the Pediatric PIVOT project, including Emily Jocoy, Ph.D., manager of JAX’s PDX resource. The Seven Bridges team will be led by Dennis Dean, Ph.D.
About JAX
The Jackson Laboratory is an independent, nonprofit biomedical research institution with more than 2,400 employees. Headquartered in Bar Harbor, Maine, it has a National Cancer Institute-designated Cancer Center, a genomic medicine institute in Farmington, Conn., and facilities in Ellsworth and Augusta, Maine, in Sacramento, Calif., and in Beijing and Shanghai, China. Its mission is to discover precise genomic solutions for disease and empower the global biomedical community in the shared quest to improve human health. For more information, please visit www.jax.org.
The JAX Cancer Center (JAXCC) is a National Cancer Institute-designated Cancer Center complemented by institutional education, resource and service initiatives that support cancer research worldwide. A major barrier to improving cancer treatment is the genetic complexity of cancer and the genetic diversity of cancer patients. The multitude of mutations found in each cancer type limits the development of a common therapeutic, and cancer’s genetic diversity and instability leads to resistance to therapies. Moreover, genetic diversity among patients contributes to varied responses to any single treatment. At the JAXCC, we are developing strategies to convert this problem of genetic complexity into an advantage in therapeutic discovery.