Mouse strains mirror variable human cognitive decline with aging.
Aging brings with it many changes, including cognitive decline. But in some people the decline is barely noticeable and they stay quite mentally sharp throughout their lives, while in others it’s more pronounced. So what happens in our brains to bring about such decline and why does it vary so much between individuals, even without underlying disorders such as Alzheimer’s disease and other dementias?
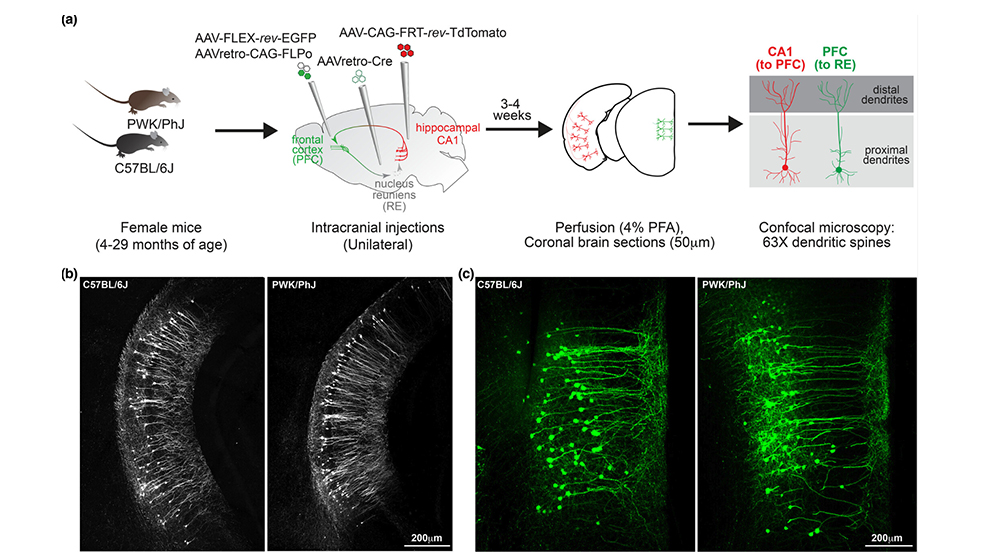
To study both questions, a research team at The Jackson Laboratory, led by Assistant Professor Erik Bloss, Ph.D., Professor Gareth Howell, Ph.D., and predoctoral associate Sarah Heuer, examined the effects of aging in mouse neurons and synapses. But instead of focusing on a single strain, the much-studied C57BL/6J (B6), which is susceptible to age-related cognitive decline, the researchers included a genetically distinct wild-derived mouse strain, PWK/PhJ (PWK). Unlike B6, PWK mice have been found to be resilient to cognitive decline when carrying Alzheimer’s disease-associated genetic mutations. The results, presented in “Genetic context drives age-related disparities in synaptic maintenance and structure across cortical and hippocampal neuronal circuits,” published in Aging Cell, show that PWK mice are also resistant to normal age-related synaptic loss compared with their B6 counterparts. The findings provide intriguing clues to what may underlie cognitive decline and, perhaps, how to promote successful cognitive aging.
Synapses forming neural circuits
While the vast majority of the approximately 3 billion base pairs in mouse genomes are the same between strains, about 20 million differences (e.g., single nucleotide polymorphisms, structural variants) have been identified between B6 and PWK mice. Recent research has shown that the differences lead to important variation in certain traits, including response to SARS-CoV-2 infection and, as stated previously, susceptibility or resistance to cognitive decline. The team therefore examined aging-related synapse loss in both strains using viral labeling strategies. Because different areas of the brain have been shown to have variable aging properties, they investigated two different neural circuits, one projecting from the hippocampal area CA1 to the prefrontal cortex (CA1-to-PFC) and the other from the PFC to the nucleus reuniens (PFC-to-RE).
 (l to r) The Jackson Laboratory's Eric Bloss, Sarah Heuer and Gareth Howell. Photo credit: Tiffany Laufer
(l to r) The Jackson Laboratory's Eric Bloss, Sarah Heuer and Gareth Howell. Photo credit: Tiffany Laufer
Synapses are the sites of communication in the nervous system; they integrate biochemical and electrical signals between neurons and shape the computations performed by neural circuits. They’re also plastic, meaning they can be altered in response to experience. Synapses on the surface of many neuronal cell types take form as dendritic spines and can be readily visualized and assessed for number and density, which are measures of synapse strength. The researchers therefore assessed synapses in the two neural circuits at three age time points: young (4–7 months), middle-aged (11–17 months) and aged (22–30 months).
The CA1-PFC network looked similar between the B6 and the PWK mice, with neurons from both strains having stable spine densities with aging. Upon closer inspection, however, the PWK dendritic spines were relatively dynamic compared to the B6 ones, undergoing age-dependent remodeling. In the PFC-to-RE circuit, dendritic spine density decreased significantly with age in B6 but not in PWK mice, indicating greater resilience to the effects of aging in the PWK strain. Also, the remaining dendritic spine structures in the B6 mice remained stable, while those in the PWK mice were more dynamic, with shapes altered to drive a predicted increase in electrical signaling. Such age-related synaptic adaptations may underlie the strain’s resistance to age-related cognitive decline.
Healthy cognitive aging?
The study points to exciting next steps for research. What cellular and molecular mechanisms underlie the loss of synapses in B6 mice over time and what drives the adaptations that make them more efficient with age in PWK mice? The answers could provide preventative and therapeutic targets for bolstering synapse retention and strength in aging adults. They also have the potential to increase resilience to Alzheimer’s disease and other dementias in susceptible individuals.