Mitochondria, for most people last seen in a textbook as the vaguely kidney bean-looking “powerhouses of the cell,” are in fact far more multifaceted and dynamic than high school biology taught us.
While they do indeed play critical roles in energy generation, they are also integral to other vital functions, including programmed cell death, molecular signaling and immunity. It’s therefore not surprising that mitochondrial function has profound effects on human health.
A. Phillip West, Ph.D., investigates mitochondria, with a focus on their roles in immunity and contributions to disease. Research has revealed that mitochondria contribute substantially to regulating the innate immune response, the front-line family of cells (e.g., macrophages, monocytes, dendritic cells, neutrophils) and signaling pathways that mount an immediate protective response against foreign materials and microbes, including pathogens. Innate immunity is crucial for health, but it operates within a system of rigorous checks and balances, and dysregulation can lead to harmful inflammation. West is studying exactly how mitochondria help to maintain carefully regulated innate immune activity and the steps that can lead to inflammation and associated diseases.
Identifying inflammatory mediators hidden within mitochondria
During infection, the innate immune system senses bacterial and viral molecules, then triggers inflammatory responses that help clear the infection. As mitochondria evolved from bacteria, many of their internal components share similarities with bacterial proteins, lipids or nucleic acids. These molecules can leak out of damaged or dysfunctional mitochondria and activate the innate immune system to promote inflammatory cascades. Prior work from West discovered that the circular mitochondrial genome, or mtDNA, is a potent inflammatory molecule when released into the cytoplasm, outside of the cell or into circulation. In recent years, West and his team have studied how mtDNA is sensed by the innate immune system and investigated how the ensuing inflammatory responses contribute to disease and aging.
“There is a growing appreciation that mitochondrial dysfunction is an important driver of inflammation,” West said. “My lab is working to understand mitochondrial—immune crosstalk to hopefully identify new therapeutic targets for mitigating chronic inflammation in disease and aging.”
 A group shot of the West Lab: (L-R) Holly White, Jordyn Van Portfliet, Abate Bashaw, Phillip West, Debi Foster, Abigail Schoeller. Photo credit: Tiffany Laufer
A group shot of the West Lab: (L-R) Holly White, Jordyn Van Portfliet, Abate Bashaw, Phillip West, Debi Foster, Abigail Schoeller. Photo credit: Tiffany Laufer
Mitochondrial dysfunction and inflammation in cancer therapy-related heart failure
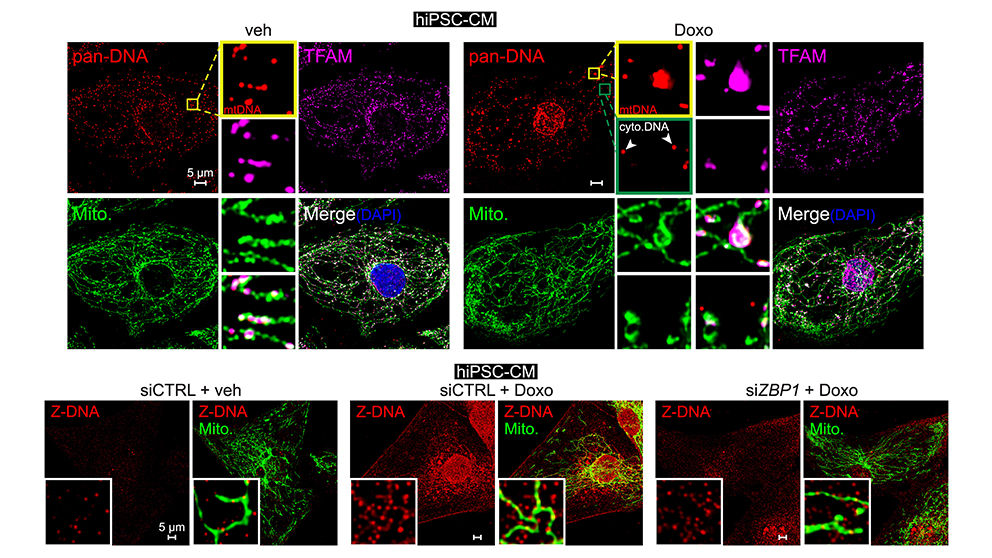
A frequent and poorly understood side effect of some cancer chemotherapies is heart damage, or cardiotoxicity. Mitochondrial dysfunction is thought to play a major role in heart failure after chemotherapy. West and his lab team have been exploring the hypothesis that mitochondrial injury may promote the release of mitochondrial contents, triggering cardiac inflammatory responses. In a recent paper published in Cell, “Cooperative sensing of mitochondrial DNA by ZBP1 and cGAS promotes cardiotoxicity,” Dr. West and his lab revealed the mitochondria-associated mechanisms underlying heart damage and failure, a severe side effect in some patients after treatment with a first-line cancer chemotherapy, Doxorubicin. Disruption to mtDNA induces a complex pathway that ultimately leads to toxic immune signaling and cardiomyocyte (heart cell) death. The work uncovered a new innate immune sensor of cardiac mtDNA damage and release, called Z-DNA Binding Protein 1 (ZBP1). Notably, experimental mouse models lacking ZBP1 were significantly protected from Doxorubicin-associated heart failure. The results indicate that disrupting this pathway could provide a new therapeutic path for oncologists to prevent Doxorubicin-associated heart failure in their patients.
West has joined The Jackson Laboratory (JAX) as associate professor after several years on the faculty at Texas A&M School of Medicine. At JAX, West works with fellow faculty in JAX’s NCI-designated Cancer Center and leverages JAX’s extensive cancer, immunology and disease modeling research resources.
“JAX provides an ideal environment to grow my research program,” says West. “Mitochondrial dysfunction and inflammation are increasingly linked to cancer, Alzheimer’s disease and other neurological disorders, and the aging process. JAX has a collaborative and dynamic faculty with significant strengths in all of these research areas. I am excited to work with labs across the institution to expand knowledge about how mitochondrial function and dysfunction shape immune responses in disease.”