With the support from the Human Frontier Science Program, J. Travis Hinson, M.D., aims to determine the fundamental biophysical and structural principles that form an important component of muscle, the sarcomere, during biogenesis.
A world in motion
Kicking a soccer ball. Hugging a friend. Swimming in the lake. Movement is a basic aspect of life. As vertebrates, we rely on skeletal and cardiac muscles for all the voluntary and involuntary movements needed throughout the day. But we often don’t think about how it is accomplished. Our muscles are formed by a multitude of cells containing intricate multiprotein complexes that enable action and movement.
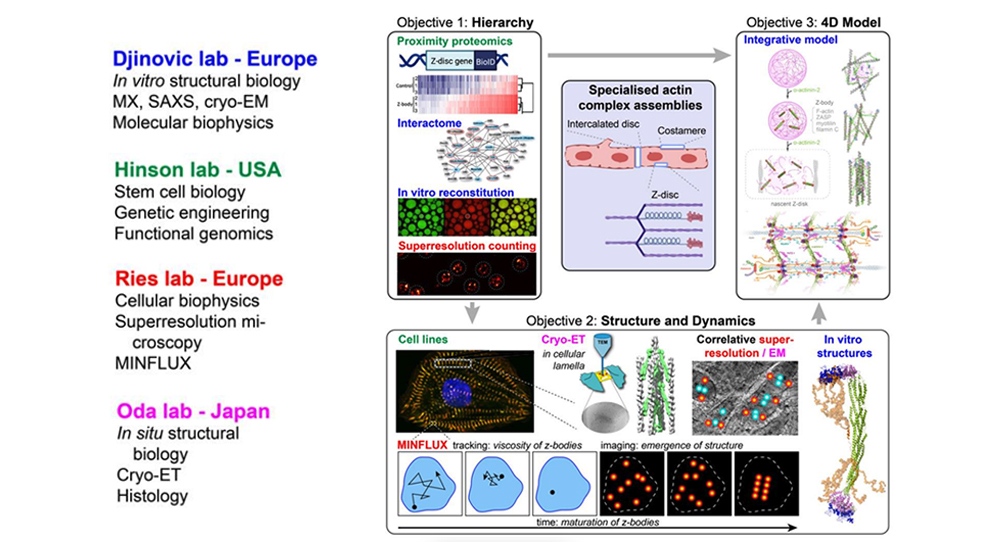
One structural aspect of muscle, the sarcomere, is of particular interest to The Jackson Laboratory (JAX) Associate Professor, J. Travis Hinson, M.D., as he and his team investigate how our genetic make-up plays a role in cardiovascular health. Sarcomeres are striated tube-like structures that play a critical role in converting molecular interactions into efficient muscle contraction. The lateral boundaries between adjacent sarcomeres are known as Z-discs, which organize, stabilize and fine tune the mechanical properties of the thin filaments comprising the muscle. Relatively little is known about how Z-discs form and the hub of protein interactions in which they reside. The Hinson lab plans to change that. Receiving support from the Human Frontier Science Program (HFSP), Hinson seeks to unravel the fundamental elements of Z-discs to help determine how the sarcomere is assembled within the cytoskeleton and how genetic variants play a role in altering its capabilities.
 Travis Hinson (l) and his lab group.
Travis Hinson (l) and his lab group.
Going global
The HFSP has equipped Hinson and his team to take part in a cross-continent collaboration with investigators in France, Germany and Japan. One of their goals is to clarify why certain protein interactions take precedent over others within the Z-disc and intercalated discs. Hinson’s team will develop several types of cellular models to help distinguish the structure and dynamics of the sarcomere during different stages of development. Part of their plan is to formulate 4D integrative molecular models that replicate sarcomeres in unprecedented accuracy by integrating biophysical rules, molecular components and regulators.
These collective efforts are paramount to unraveling the complexities of sarcomere assembly, not only for those with full muscular function, but also for those with muscular impairments or disease.