Sheng Li explores how aging raises the risk for leukemia, and works to improve tools for data analysis.
As people age, their risk for deadly leukemia rises. Sheng Li wants to understand the link between acute myeloid leukemia and the changes that occur in blood as we age, with the goal of making this cancer less likely.
Most biomedical researchers are either “wet-lab” (those who study diseases and biological processes in cells or animal models) or “dry-lab,” focused on computation and data analysis. Li’s multidisciplinary work puts her in both categories.
“My lab is at the interface between computation and biology,” she says. “We design and generate these large, complex datasets in our work to understand how aging contributes to leukemia. Then, we get to explore these datasets, using new, deep-learning algorithms and statistical algorithms to discover clues to the mechanisms involved.”
Li’s interest in improving data analysis techniques extends beyond her own laboratory’s findings. Her latest paper, currently the featured article in Genome Biology, systematically benchmarks computational methods that the scientific community uses to detect DNA modifications from long-read sequencing data. The cover art of the paper was selected for the journal’s Long Read Sequencing Collection.
A scientific upbringing
Li was in elementary school when she first learned computer programming. “It was simple code to make a triangle move forward and turn,” Li says, “and it was fun.”
Growing up, Li recalls, her parents gave her the freedom to do what she liked, which included lots of reading. “Since elementary school, I’ve read a lot of fiction and nonfiction books, including the biographies of extraordinary scientists whose passion and dedication are inspirational.”
Li earned a bachelor’s degree in biotechnology from Sun Yat-sen University in Guangzhou, China, where she was fascinated by microarray technology to measure the expression of thousands of genes in a single assay. She came to Cornell University in New York for her Ph.D. studies in computational biology in the lab of Christopher Mason, where she returned to her early interest in programing and its application to next-generation (“next-gen,” in lab parlance) sequencing data. She joined JAX in 2016 from Weill Cornell Medicine in New York, where she was an instructor of bioinformatics working in cancer epigenome dynamics.
Appropriately, last year the American Association for Cancer Research named Li among 12 “NextGen Stars”, early-career researchers recognized for their outstanding work and future potential.
By every measure of researcher success, Li is building an impressive track record at JAX. She has authored or coauthored articles in several prestigious journals, including Cancer Discovery, and Nature Immunology. Since 2019, Li has been awarded two multi-year federal research grants.
Aging and leukemia: the epigenetic connection
As people age, Li explains, “the chance of getting cancer, including leukemia, increases dramatically. So basically, we’re trying to understand how the aging process contributes to leukemia initiation.”
Acute myeloid leukemia is a deadly blood cancer that occurs mostly in elderly people and is associated with the abnormal over-proliferation of hematopoietic stem and progenitor cells (HSPCs), the blood-generating cells in the bone marrow. This common, age-related condition called clonal hematopoiesis (CH) results from the expansion of HSPCs carrying mutations in leukemia-associated genes. If you are over 50, you likely have this age-related CH.
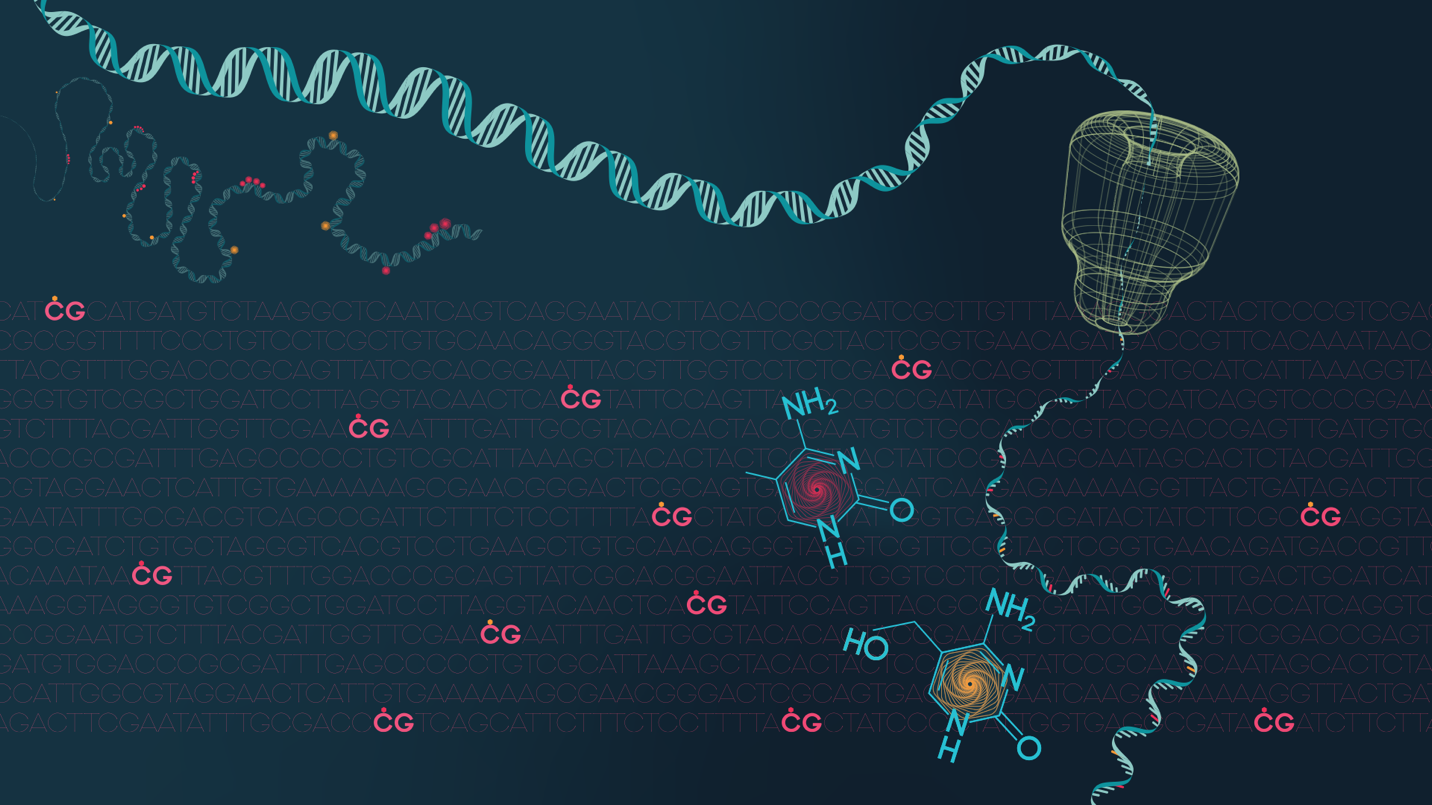
In general, aging and cancer are closely linked because molecular lesions accumulate in our cells as we age. Some of these lesions are mutations that alter the sequence of DNA in ways that may lead to cancer. Others, known as epigenetic modifications, leave the sequence alone but change how genes function (silencing or activating), another potential path to cancer.
Li explores lesions that tick both boxes. She investigates the impact of mutations in epigenetic modifiers and their impact on epigenome and transcriptome – more than two thirds of AML patients carry these mutations. These mutations are involved with DNA demethylation, an epigenetic process that is vital for early mammalian development but potentially cancer-causing in adult cells.

In October 2021, Li was awarded a five-year grant totaling $2 million from the National Cancer Institute to elucidate how aging and CH influence the propensity of blood stem cells to adapt to altered environments — which she refers to as “evolvability” — which could not only forge a new paradigm for understanding leukemia onset but also provide candidate pathways for the development of preventative interventions.
“We seek to reveal the epigenetic mechanisms for how CH evolves to leukemia in an aging microenvironment,” Li says, “laying the foundation for therapeutic strategies to block this evolution and prevent leukemia in the aging U.S. population.”
Li’s co-principal investigator on the grant is James DeGregori, professor in biochemistry and medical genetics in the University of Colorado’s Anschutz Medical Campus in Aurora, and an expert in the evolutionary biology of hematopoietic malignancies. “Sheng really is an ideal collaborator,” DeGregori says. “She’s incredibly energetic and super talented. With Sheng at the helm, I have no doubt that we will make important new discoveries on this project, that will eventually impact people’s lives. And we’ll have fun along the way.”
Together with co-investigators Eric Pietras, also from the University of Colorado, and Hideyuki Oguro of the University of Connecticut Health Center, the team will combine genetic barcoding and single-cell multi-omics to crack the code of aging and cancer. Notably, they are among the eleven teams in the Onco-Aging Consortium, jointly funded by the National Cancer Institute and the National Institute on Aging.
Looking for ways to slow down the aging process
In July 2019, the National Institute of General Medical Sciences announced funding to Li for a five-year project that will support her “dry lab” work. The Li lab has been developing computational tools to define how two types of epigenetic modifications, called methylation and hydroxymethylation, jointly impacts gene expression and ultimately cell behavior. The grant is from a special category, “Maximizing Investigators’ Research Award,” aimed at supporting exceptionally talented and promising early-stage researchers. Li’s work also received support from two JAX internal funding sources, the Cancer Center Fast Forward Program and the Director’s Innovation Fund.
“These tools will be versatile and adaptable to any cell type of choice, enabling research into the molecular features of the genome that underlie both normal and pathological cell behaviors,” Li says. She sees the computational project as directly supporting her blood cancer research. “The advanced computational tools can be applied to analyze mutations in those cells from aging individuals.”
What drew Li to cancer research? “When I was at Cornell, I had the opportunity to talk to top leukemia biologists like Drs. Ari Melnick and Ross Levine. They taught me to think about how I can better use computational analysis and to look at data in new and different ways.”
Young as she is, Li says, “We are all getting old, right? And with the whole human population aging, cancer is just inevitable. I'm on the path of looking for ways to slow down the aging process and expand health span, so we can live not just longer but healthier lives free of cancer. And that's something that is of value to myself, my family and everyone.”