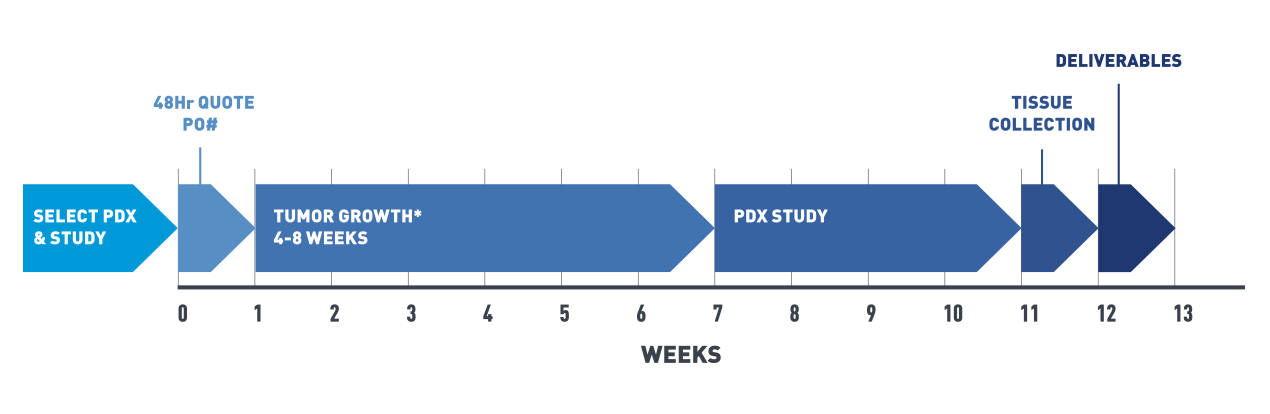
Fast-track your efficacy testing projects
Our In Vivo Pharmacology Services now offers optimized Patient Derived Xenograft (PDX) efficacy studies utilizing its PDX Live™ library. These select tumors are kept at low passage in live donor mice, enabling fast-tracked efficacy testing studies for pre-clinical cancer drug development, potentially saving months of pre-study time.

PDX-engrafted mice are a fast and cost-effective platform to simulate trials, evaluate multiple drugs alone or in combination, and produce predictive data.